When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital.
- Formula: Cl
- Molecular weight: 35.453
- IUPAC Standard InChI:
- InChI=1S/Cl
- Download the identifier in a file.
- IUPAC Standard InChIKey:ZAMOUSCENKQFHK-UHFFFAOYSA-N
- CAS Registry Number: 22537-15-1
- Chemical structure:
This structure is also available as a 2d Mol file - Permanent link for this species. Use this link for bookmarking this speciesfor future reference.
- Information on this page:
- Other data available:
- Data at other public NIST sites:
- Options:
Data at NIST subscription sites:
NIST subscription sites provide data under theNIST Standard ReferenceData Program, but require an annual fee to access.The purpose of the fee is to recover costs associatedwith the development of data collections included insuch sites. Your institution may already be a subscriber.Follow the links above to find out more about the datain these sites and their terms of usage.
- Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature.
- Chlorine atom Please visit the Chlorine element page for information specific to the chemical element of the periodic table.
- Chlorine has two stable isotopes chlorine-35 and chlorine-37with Chlorine-35 accounting for roughly 3 out of every 4 naturally occurring chlorine atoms. Chlorine-36 is also known naturally and is a radioactive isotope with a half life of about 30,000 years.
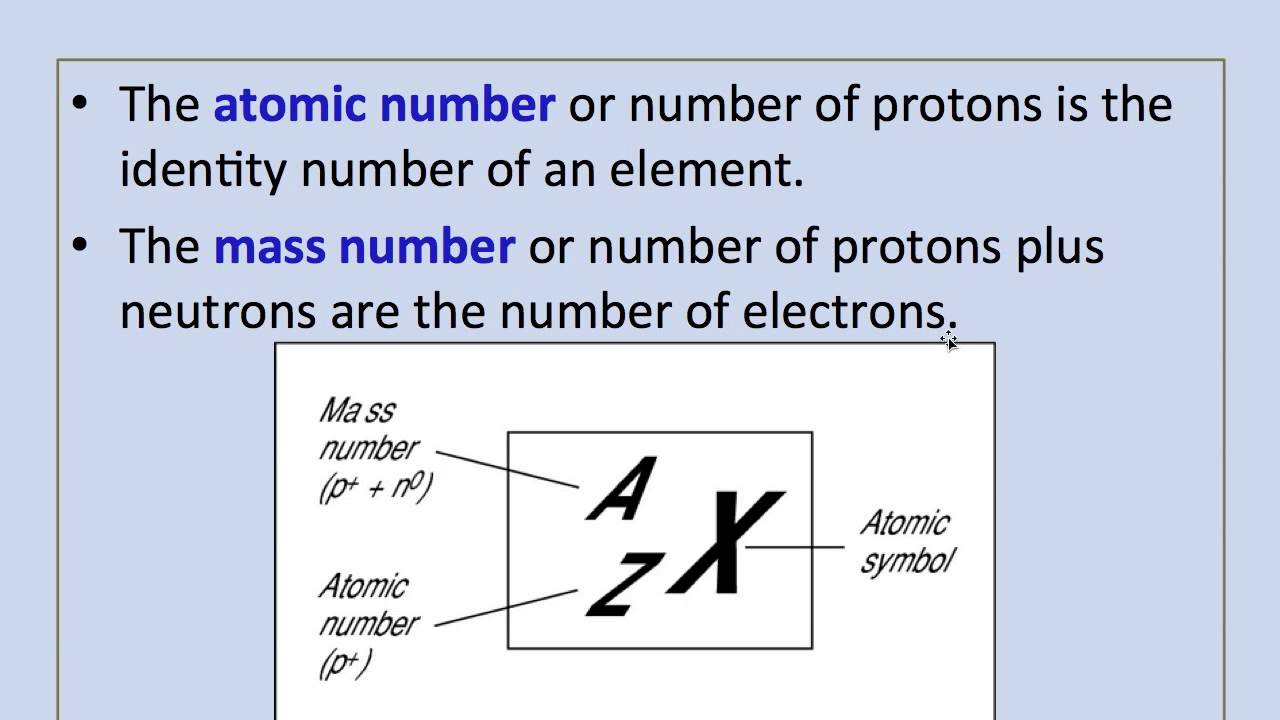
- The total number of electrons in a chlorine atom is 17, which is also the atomic number of that element. Of those, 7 are outer (aka valence) electrons, having 3 as principal quantum number. The other 10 electrons, having principal quantum number l.
Gas phase thermochemistry data
Chlorine Atomic Number
Go To:Top, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔfH°gas | 121.301 ± 0.008 | kJ/mol | Review | Cox, Wagman, et al., 1984 | CODATA Review value |
| ΔfH°gas | 121.30 | kJ/mol | Review | Chase, 1998 | Data last reviewed in June, 1982 |
| Quantity | Value | Units | Method | Reference | Comment |
| S°gas,1 bar | 165.190 ± 0.004 | J/mol*K | Review | Cox, Wagman, et al., 1984 | CODATA Review value |
| S°gas,1 bar | 165.19 | J/mol*K | Review | Chase, 1998 | Data last reviewed in June, 1982 |
Gas Phase Heat Capacity (Shomate Equation)
Cp° = A + B*t + C*t2 + D*t3 + E/t2
H° − H°298.15= A*t + B*t2/2 + C*t3/3 + D*t4/4 − E/t + F − H
S° = A*ln(t) + B*t + C*t2/2 + D*t3/3 − E/(2*t2) + G
Cp = heat capacity (J/mol*K)
H° = standard enthalpy (kJ/mol)
S° = standard entropy (J/mol*K)
t = temperature (K) / 1000.
View plotRequires a JavaScript / HTML 5 canvas capable browser.
View table.
| Temperature (K) | 298. - 600. | 600. - 6000. |
|---|---|---|
| A | 13.38298 | 23.26597 |
| B | 42.33999 | -1.555939 |
| C | -64.74656 | 0.346910 |
| D | 32.99532 | -0.025961 |
| E | 0.063319 | 0.153212 |
| F | 116.1491 | 114.6604 |
| G | 171.7038 | 193.8882 |
| H | 121.3021 | 121.3021 |
| Reference | Chase, 1998 | Chase, 1998 |
| Comment | Data last reviewed in June, 1982 | Data last reviewed in June, 1982 |
References
Go To:Top, Gas phase thermochemistry data, Notes
Chlorine Atom Draw
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Cox, Wagman, et al., 1984
Cox, J.D.; Wagman, D.D.; Medvedev, V.A.,CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp., New York, 1984, 1. Videopad video editor code. [all data]
Chlorine Periodic Table
Chase, 1998
Chase, M.W., Jr.,NIST-JANAF Themochemical Tables, Fourth Edition,J. Phys. Chem. Ref. Data, Monograph 9, 1998, 1-1951. [all data]
Notes
Go To:Top, Gas phase thermochemistry data, References
Chlorine Atomic Radius
- Symbols used in this document:
S°gas,1 bar Entropy of gas at standard conditions (1 bar) ΔfH°gas Enthalpy of formation of gas at standard conditions - Data from NIST Standard Reference Database 69:NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST)uses its best efforts to deliver a high quality copy of theDatabase and to verify that the data contained therein havebeen selected on the basis of sound scientific judgment.However, NIST makes no warranties to that effect, and NISTshall not be liable for any damage that may result fromerrors or omissions in the Database.
- Customer supportfor NIST Standard Reference Data products.
